242
Members
11
Research Team
75
Researchers
69
Engineers, Technicians, Administrative staff
CIIL'S RECENT PUBLICATIONS
-
Plasma albumin contributes to early Yersinia pestis survival at the onset of flea infection
-
Accumulation of Quinolinic Acid Modulates the Pulmonary Immune Response During Influenza…
-
Optimizing the Antibiotic Potency and Metabolic Stability of Pyridomycin Using a Semisynthetic…
-
A new family of TonB-dependent copper transporters linked to respiratory oxidase function
-
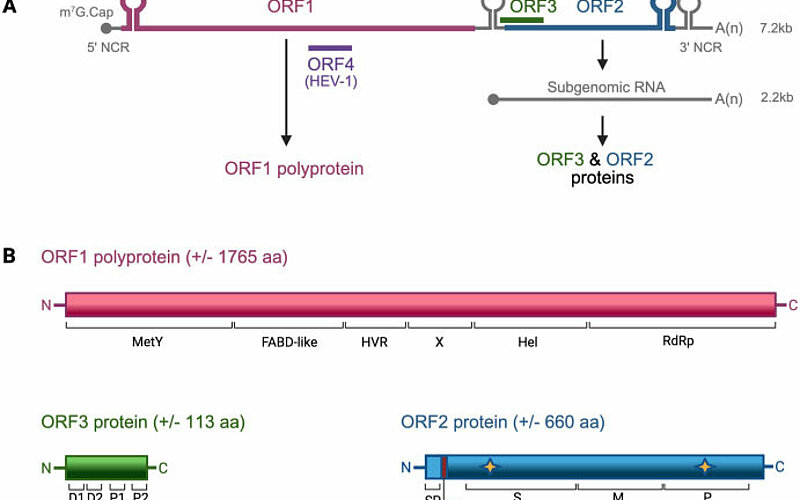
Update on the molecular and cellular biology of hepatitis E virus and therapeutic…
CIIL'S LATEST NEWS
-
 Ideas, talent and community: our 2025 off-site day
Ideas, talent and community: our 2025 off-site day -
 Engaged in field research with Dr. Fernando Real
Engaged in field research with Dr. Fernando Real -
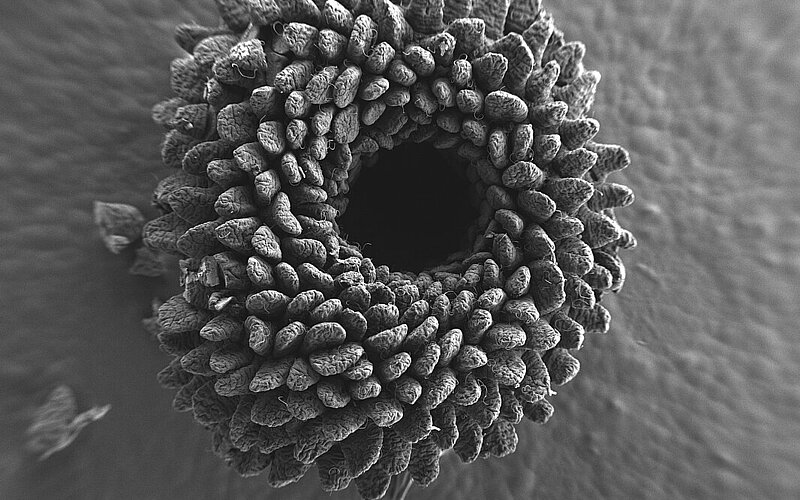 Nicolas Barois, affiliated with CIIL U1019 (UMR 9017) / BICeL Platform, UAR 2014 – US 41, PLBS (Lille Platforms in Biology and Health), has been awarded First Prize in the 2025 FBI Image Contest.
Nicolas Barois, affiliated with CIIL U1019 (UMR 9017) / BICeL Platform, UAR 2014 – US 41, PLBS (Lille Platforms in Biology and Health), has been awarded First Prize in the 2025 FBI Image Contest. -
 The awarding of the poster winner has been attributed to Joan while the 7th European Congress of Immunology
The awarding of the poster winner has been attributed to Joan while the 7th European Congress of Immunology -
 Success stories
Success stories












