Antibacterial Drug Discovery & Development

Ruben C. HARTKOORN
Research Director (INSERM)
Antimicrobial resistance is escalating at an alarming rate, undermining the effectiveness of existing antibiotics and making once-treatable infections harder and sometimes impossible to cure. Currently world-wide around 5 million die yearly from infections associated with drug resistant bacteria, a number predicted to escalate to 39 million over the next decades. At the forefront of this crisis are infections by drug resistant bacteria highlighted by the WHO priority list, bacteria that are not only very difficult to treat, but also for which the discovery of novel effective antibiotics is particularly challenging. The discovery and development of new treatments for such bacterial infection is urgently needed to save lives and to prevent everyday infections and routine medical procedures to once again become life-threatening.
The AntiBacterial Drug Discovery and Development (AntiBac) team, headed by Dr. Ruben Hartkoorn, is committed to addressing this critical global challenge by discovering new ways to treat infections caused by antibiotic-resistant bacteria. To drive innovation in this field, the team focuses on discovering and developing new antibiotics, as well as novel molecules that can restore the effectiveness of existing treatments by overcoming antibiotic resistance, with the goal of developing them from the lab toward clinical use. As an example, the team and collaborators currently conducts R&D on novel antibiotics for the treatment of drug resistant Mycobacteria (M. tuberculosis, M. abscessus and M. avium), novel efflux pump inhibitors to revert antibiotic resistance in Enterobacterales (E. coli. K. pneumoniae) and biomimetic vectorisation of antibiotics to improve uptake and activity (P. aeruginosa, K. pneumoniae, E. coli, A. baumannii, M. abscessus).
More details are available in the projects tab.
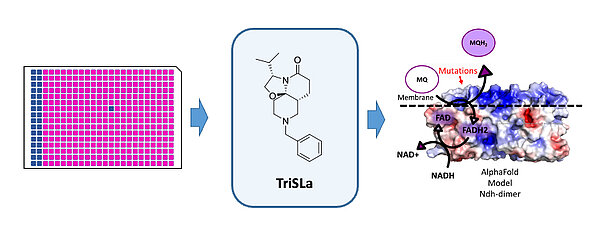
The primary research programs of the AntiBac team are currently centered on 1) the collaborative development of TricyclicSpiroLactam (TriSLa) based inhibitors as a novel class of anti-mycobacterial drug, 2) the development of Trojan horse antibiotics using novel biomimetic siderophore-antibiotic conjugates, and 3) the collaborative development of pyridylpiperazine based efflux pump inhibitors as an adjuvant therapy for the antibiotic treatment of Enterobacterales infections.
Development of a novel anti-tuberculosis drug
Tuberculosis treatment is very lengthy and further exacerbated for prevalent multi-drug resistant strains. To feed the drug development pipeline for the antibiotic treatment of tuberculosis, the AntiBac team and its collaborative partners in medicinal chemistry (particularly Dr. B. Villemagne and Pr. N. Willand, U1177, https://www.deprezlab.fr/) have discovered and characterised a novel class of anti-tuberculosis molecules named TricyclicSpiroLactams (TriSLas) that are potent inhibitors of the type II NADH dehydrogenase of the bacterial electron transport chain (doi:10.1021/acs.jmedchem.2c01493). Current collaborative research efforts are focused on the optimisation of TriSLa drug like properties (ADMET/PK), characterising TriSLa efficacy in infection models, developing and evaluating the efficacy of TriSLa based combination regimens and characterising the antibiotic efficacy of TriSLa against non-tuberculous mycobacteria such as M. abscessus and M. avium.
Key AntiBac team members involve: Dr. Vien Ho, Dr. Kamel Djaout, Jonathan Chatagnon, Hind Oulkfif, Dr. Elizabeth Pradel and Dr. Ernesto Anoz-Carbonell (Past: Dr Sushovan Dam)
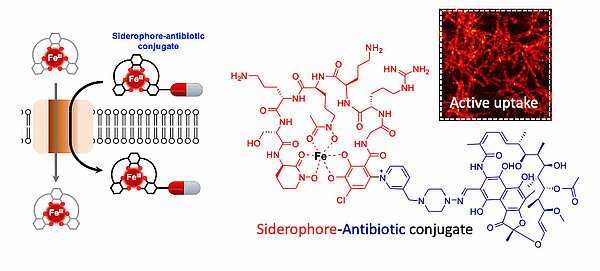
Novel biomimetic Trojan horse antibiotics
The complex cell wall of bacteria is a formidable xenobiotic penetration barrier and a major hurdle in antibiotic drug development. One approach observed in nature to overcome this penetration barrier is to hijack the iron acquisition machinery of bacteria for uptake. To acquire iron, bacteria synthesise and export small molecules, called siderophores. Siderophores effectively scavenge iron from the environment and are then actively taken up to internalise the iron. Some bacterial natural products are composed of an antibiotic coupled to a siderophore. In the bacterial interspecies warfare, these molecules hijack the iron uptake machinery of another species to achieve intracellular antibiotic delivery. Such natural systems have inspired the chemical synthesis of mimetic siderophore-antibiotic conjugates with improved bacterial penetration. In the AntiBac lab, studies on antibiotic production by a soil bacterium led to the discovery of a new siderophore-antibiotic conjugate, and importantly, identified a novel means of coupling siderophores to antibiotics (doi:10.1021/acscentsci.3c00965). In an ERC-COG supported program named Antibioclicks, this mechanism of macromolecular conjugation is now being exploited to generate, characterise and develop bioinspired Trojan horse antibiotics to target the WHO priority bacterial pathogens.
Key AntiBac team members involve: Dr. Ernesto Anoz-Carbonell, Dr. Ravil Petrov, Dr. Shahriar Safari (Past: Dr. Thibault Caradec & Dr Iullia Ustimenko)
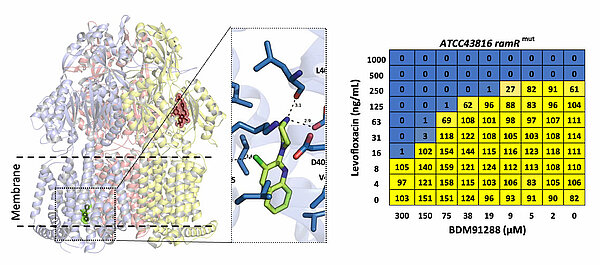
Antibiotic efflux pump inhibitors as antibiotic resistance breakers
In addition to their physiological role, bacterial efflux pumps are extremely efficient at expelling antibiotics out of bacteria. Efflux pumps are a major cause of both innate antibiotic resistance (particularly in Gram-negative bacteria) as well as acquired antibiotic drug resistance. Efflux pump inhibitors (EPIs) could serve as adjuvants to improve antibiotic activity, and fight acquired drug resistance). In a collaborative program with medicinal chemists (particularly Dr. M. Flipo, U1177, https://www.deprezlab.fr/), the national reference centre for Enterobacterales (Dr. R. Bonnet) and infection model specialist, Dr. L. Van Maele (CIIL), the AntiBac team is developing a new class of Pyridylpiperazines (PyrPips) based EPIs that are potentiators of antibiotic activity in Enterobacterales (doi: 10.1038/s41467-021-27726-2, 10.1093/jacamr/dlad112, 10.1016/j.ejmech.2023.115630, 10.1038/s44321-023-00007-9). Current effort focus on deciphering the in vitro and in vivo impact of EPIs on the treatment of multidrug-resistant clinical strains, with the aim of defining the optimal means of using such EPIs to revert antibiotic resistance.
Key AntiBac team members involve: Dr. Elizabeth Pradel, Cécilia Sobieski, Léa Lèbre, Jonathan Chatagnon (Past: Dr. Coline Plé, Dr. Juan Carlos Jiménez-Castellanos)
Current Staff
Ernesto Anoz Carbonell
INSERM researcher (CRCN)
Ernesto's ORCID
Ernesto's contact
Jonathan Chatagnon
Engineer (IPL)
Jonathan's contact
Kamel Djaout
Research engineer (Inserm)
Kamel's ORCID
Kamel's contact
Ruben Hartkoorn
Inserm researcher director
Ruben's ORCID
Ruben's contact
Learn more about Ruben
Lea Lebre
Engineer (IE)
Lea's contact
Hind OULKFIF
PhD Student, University of Lille
Hind's contact
Ravil Petrov
Postdoctoral researcher
Ravil's ORCID
Ravil's contact
Elizabeth Pradel
Inserm Researcher
Elizabeth's ORCID
Elizabeth's contact
Vien QT Ho
Postdoctoral researcher
Vien's ORCID
Vien's contact
Shahriar Safari
Postdoctoral researcher
Safari's contact
Cecilia Sobieski
PhD Student, University of Lille
Cecillia's contact
- Caradec T, Plé C, Sicoli G, Petrov R, Pradel E, Sobieski C, Antoine R, Orio M, Herledan A, Willand N, Hartkoorn RC. Small molecule MarR modulators potentiate metronidazole antibiotic activity in aerobic E. coli by inducing activation by the nitroreductase NfsA. J Biol Chem. 2024 Jul;300(7):107431. doi: 10.1016/j.jbc.2024.107431. Epub 2024 May 31. PMID: 38825006; PMCID: PMC11259696.
- Vieira Da Cruz A, Jiménez-Castellanos JC, Börnsen C, Van Maele L, Compagne N, Pradel E, Müller RT, Meurillon V, Soulard D, Piveteau C, Biela A, Dumont J, Leroux F, Deprez B, Willand N, Pos KM, Frangakis AS, Hartkoorn RC, Flipo M. Pyridylpiperazine efflux pump inhibitor boosts in vivo antibiotic efficacy against K. pneumoniae. EMBO Mol Med. 2024 Jan;16(1):93-111. doi: 10.1038/s44321-023-00007-9. Epub 2023 Dec 20. PMID: 38177534; PMCID: PMC10897476.
- Caradec T, Anoz-Carbonell E, Petrov R, Billamboz M, Antraygues K, Cantrelle FX, Boll E, Beury D, Hot D, Drobecq H, Trivelli X, Hartkoorn RC. A novel natural siderophore antibiotic conjugate reveals a chemical approach to macromolecule coupling. ACS Cent Sci. 2023 Nov 10;9(11):2138-2149. doi: 10.1021/acscentsci.3c00965. PMID: 38033789; PMCID: PMC10683483.
- Jiménez-Castellanos JC, Pradel E, Compagne N, Vieira Da Cruz A, Flipo M, Hartkoorn RC. Characterization of pyridylpiperazine-based efflux pump inhibitors for Acinetobacter baumannii. JAC Antimicrob Resist. 2023 Oct 24;5(5):dlad112. doi: 10.1093/jacamr/dlad112. PMID: 37881353; PMCID: PMC10594211.
- Compagne N, Jiménez-Castellanos JC, Meurillon V, Pradel E, Vieira Da Cruz A, Piveteau C, Biela A, Eveque M, Leroux F, Deprez B, Willand N, Hartkoorn RC, Flipo M. Optimization of pyridylpiperazine-based inhibitors of the Escherichia coli AcrAB-TolC efflux pump. Eur J Med Chem. 2023 Nov 5;259:115630. doi: 10.1016/j.ejmech.2023.115630. Epub 2023 Jul 7. PMID: 37459793.
- Caradec T, Trivelli X, Desmecht E, Peucelle V, Khalife J, Hartkoorn RC. Dactylosporolides: glycosylated macrolides from Dactylosporangium fulvum. J Nat Prod. 2022 Dec 23;85(12):2714-2722. doi: 10.1021/acs.jnatprod.2c00484. Epub 2022 Dec 13. PMID: 36512509; PMCID: PMC9791991.
- Dam S, Tangara S, Hamela C, Hattabi T, Faïon L, Carre P, Antoine R, Herledan A, Leroux F, Piveteau C, Eveque M, Flipo M, Deprez B, Kremer L, Willand N, Villemagne B, Hartkoorn RC. Tricyclic SpiroLactams kill mycobacteria in vitro and in vivo by inhibiting type II NADH dehydrogenases. J Med Chem. 2022 Dec 22;65(24):16651-16664. doi: 10.1021/acs.jmedchem.2c01493. Epub 2022 Dec 6. PMID: 36473699; PMCID: PMC9791652.
- Plé C, Tam HK, Vieira Da Cruz A, Compagne N, Jiménez-Castellanos JC, Müller RT, Pradel E, Foong WE, Malloci G, Ballée A, Kirchner MA, Moshfegh P, Herledan A, Herrmann A, Deprez B, Willand N, Vargiu AV, Pos KM, Flipo M, Hartkoorn RC. Pyridylpiperazine-based allosteric inhibitors of RND-type multidrug efflux pumps. Nat Commun. 2022 Jan 10;13(1):115. doi: 10.1038/s41467-021-27726-2. PMID: 35013254; PMCID: PMC8749003.
- Antoine R, Gaudin C, Hartkoorn RC. Intragenic distribution of IS6110 in clinical Mycobacterium tuberculosis strains: bioinformatic evidence for gene disruption leading to underdiagnosed antibiotic resistance. Microbiol Spectr. 2021 Sep 3;9(1):e0001921. doi: 10.1128/Spectrum.00019-21. Epub 2021 Jul 21. PMID: 34287057; PMCID: PMC8552512.
- Valderrama K, Pradel E, Firsov AM, Drobecq H, Bauderlique-le Roy H, Villemagne B, Antonenko YN, Hartkoorn RC. Pyrrolomycins are potent natural protonophores. Antimicrob Agents Chemother. 2019 Sep 23;63(10):e01450-19. doi: 10.1128/AAC.01450-19. PMID: 31405863; PMCID: PMC6761498.