Targeted Immunomodulation for Infection & Lung diseases

Muriel Pichavant
INSERM researcher
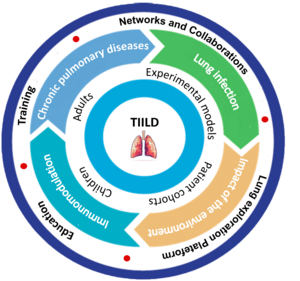
The TIILD team has been created in January 2026. The overall objective of the TIILD team is focusing on the understanding of immunopathological defects leading to the development and evolution of chronic inflammatory pulmonary diseases, namely asthma and COPD. Our team’s approach is based on a bidirectional complementarity between fundamental and translational research involving both basic and clinical researchers. The cornerstone of our project lies in a bench-to-bedside approach, where the collective expertise and knowledge of TIILD team members contribute to meaningful translational research aimed at addressing major health issues. We will test hypotheses both in in vivo mouse models and ex vivo in humans, with patient samples provided by Lille University Hospital, with the specific possibility to go from children to adults.
Chronic lung diseases are a major public health challenge, affecting millions of people worldwide. These conditions, such as asthma and COPD, are characterised by progressive deterioration of respiratory function, leading to reduced quality of life and increased morbidity and mortality. In addition, these conditions make individuals more susceptible to respiratory infections, including viral, bacterial and fungal infections, which further worsen their health and prognosis. Understanding the underlying mechanisms that increase susceptibility to infection in patients with chronic lung disease is essential for developing effective prevention and treatment strategies. This research project aims to explore in depth the complex links between chronic lung disease and susceptibility to infection, examining the immunological, environmental, genetic and behavioural factors that contribute to this interaction. It will also focus on identifying potential new therapeutic targets and developing innovative therapies to improve patient respiratory health and reduce their risk of respiratory infections. Ultimately, this research aims to make significant advances in the field of respiratory disease.
The proposed TIILD team has:
- a significant know-how in experimental models of severe asthma and COPD
- the ability to develop animal models of exacerbation of asthma and COPD (bacterial and/or viral infections)
- the knowledge and the tools to investigate immune responses
- developed alternatives models to animals, including lung organoïds and Precision-cut lung slices
- a strong translational network in pulmonology and infectious diseases
We postulate that changes in the immune system associated with chronic lung disease increase susceptibility to respiratory infections by impairing the local and systemic immune response. To explore this working hypothesis, the TIILD team will promote 5 aims, covering bench-to-bedside approaches:
Aim 1- To analyse the gene and protein expression profiles of immune cells in the lungs and airways of patients with different chronic lung diseases before and after respiratory infection (led by Cécile Chenivesse and Stéphanie Lejeune):
The goal of this aim is to identify the specific features and biomarkers of inflammation, as well as new genes, transcripts and abnormally expressed proteins or metabolites that characterise new phenotypes/endotypes of asthma and COPD patients.
Task 1.1- This work has already started with the establishment of human cohorts by clinical researchers from the TIILD team, namely Virasthma 1 and 2. Based on recent data using Omic approaches (Lejeune S, et al., J Allergy Clin Immunol. 2024), new pathways leading to asthma will be investigated. Team members are currently investing in single cell analysis and spatial biology to better decipher changes occurring between patients. A special focus will be done on the kynurenine pathway, as suggested in our last review (Pamart G, et al Int J Tryptophan Res. 2024).
Task 1.2- Since IL-10-related cytokines are significantly altered in the airways of patients with asthma and COPD, some of these proteins may serve as accessible diagnostic targets. However, it remains unclear whether the alterations in local IL-10 cytokines observed in asthma and COPD are a reactive, protective response or a truly pathogenic contributor to the progression of these airway diseases. Thanks to the cohorts involving TIILD members, we have access to human samples. Our hypothesis is that the level of one of the IL-10 cytokines will discriminate the patients susceptible to airway infection. In the end, we aim to identify distinct patient profiles, enabling the identification of potential biomarkers of host vulnerability to infections.
Aim 2- To study the functional changes of immune cells such as epithelial cells, macrophages, lymphocytes and neutrophils in the lung in response to common respiratory pathogens (led by Patricia de Nadaï and Teddy Grandjean):
This aim is strongly supported by previous works done by TIILD members on the Th17 pathway in the context of both asthma and COPD (Le Rouzic O et al., Eur Respir J. 2017). Our current understanding suggests that this group of cytokines constitutes a unique arm of the immune system that induces innate defense mechanisms in epithelial tissues, thereby protecting these tissues from damage during infection and inflammation and restoring tissue homeostasis. Previous results from TIILD members are showing that the more the chronic disease is advanced, the more the IL-10 cytokines, in particular IL-22 and IL-20, are increased in the lung mucosa, both in murine and human samples (Barada O et al., Cells. 2023; Bouté et al., Allergy. 2021).
Task 2.1- We need to determine the cellular sources of the IL-10 cytokines, including structural cells such as epithelial cells and endothelial cells, and immune cells (antigen-presenting cells, lymphocytes, neutrophils), and the target cells. This will be determined at steady-state as well as in a chronological manner in the context of chronic respiratory diseases, using our preclinical models exposed to allergens to mimic asthma, cigarette smoke to mimic COPD. This approach will also be performed after infection with pulmonary pathogens (Influenza A virus, Streptococcus pneumoniae, Pseudomonas aeruginosa). The mapping of the IL-10 cytokines will be appreciated by multiplexed imaging combining immunofluorescence and immunohistochemistry, after cell sorting and single-cell spatial analysis. Second, we will identify the factors involved in the modulation of their expression (inflammatory cytokines, TLR ligands, antimicrobial peptides) and how the expression of these cytokines is regulated.
Task 2.2- Previous work from TIILD members has demonstrated the alteration of lung antigen presenting cells. We need now to identify the specific subset of pulmonary antigen-presenting cells is playing a critical role in the susceptibility to infections. For this purpose, we will characterize the different cell subsets (spectral flow cytometry and single-cell RNA analysis after cell sorting), and try to specifically target them either by depleting or activating them.
Task 2.3- Both asthma and COPD are characterized by a strong remodelling of the airways. We, therefore, aim to investigate key-factors (including IL-20 and IL-22) on the expression of tight junction molecules, epithelial cell proliferation and differentiation, and finally on tissue remodelling and wound healing in the context of asthma and COPD. Since tissue remodelling can lead to a loss of function, the role of the identified keyplayers will be evaluated on lung function (Flexivent).
Aim 3- To investigate the mechanisms underlying immune dysfunction in chronic lung diseases, with particular emphasis on inhibition of the antimicrobial response, modulation of inflammation and disruption of the epithelial barrier (led by Silvia Gaggero, Saliha Aït-Yahia and Muriel Pichavant):
It has been described that IL-22 and IL-20 subfamily cytokines induce antimicrobial responses in epithelial and immune cells. To date, all family members, except for IL-26, promote the production of various antimicrobial peptides (AMP). We, therefore, aim to characterize the actors of the direct antimicrobial response of the IL-10 cytokine family within the lungs. Using alternative models we are developing and mimicking the airway interface, we will exploit cultures between epithelial cells and antigen presenting cells in relevant conditions. We will evaluate the levels of AMP and IL-10 cytokines, add or deplete relevant AMP to see the impact on IL-10 cytokines. The final outcome will be the levels of pathogens in these different conditions. This will help us to understand how these cytokines are involved in the clearance and/or control of pulmonary pathogens. It will also be important to further elucidate the antimicrobial factors and mechanisms down/upstream of IL-20 subfamily cytokines that may be subverted by pathogens to evade a protective immune response.
Aim 4- To assess the impact of the environment on the immune response to respiratory infections, particularly with regard to treatments and pollutants (led by Rémi Le Guern and Alexandre Gaudet):
Task 4.1 – Patients suffering from asthma and COPD are often treated with corticoids to limit inflammation. We want to investigate the impact of such a treatment on vulnerability to infections. In addition, asthma and COPD patients often receive antibiotics. However, TIILD members have shown a negative impact of antibiotic treatment on lung infection (Dessein R et al., Crit Care. 2020; Le Guern R, et al., Nat Commun. 2023). We will keep investigating how antibiotics disrupt immune homeostasis and the mechanisms leading to higher vulnerability to lung infections.
Task 4.2 – Epidemiological studies and data from TIILD members (Carrard J, et al. Allergy. 2021) have demonstrated an association between air pollutants and occurrence of lung infections. We need to further investigate the underlying mechanisms leading to a defective control of the infection. Projects are currently funded by ANSES to study the effects of climate changes on pollen and their capacity to induce allergic asthma. Private fundings obtained from Fondation Air liquide in September 2024 also allow to decipher the impact of exposure to PM2.5 on the lung immune responses leading to a defective control of respiratory infections.
Aim 5- To explore new therapeutic strategies aimed at boosting the immune response in patients with chronic lung disease to reduce their susceptibility to respiratory infections and improve their quality of life (led by Olivier Le Rouzic and Emmanuel Faure):
We will explore therapeutic approaches to limit inflammation, to modify mucosal immune responses and to restore a competent immune response to pathogens. We aim to validate the safety of our therapies in the context of chronic lung pathologies, ensuring that treatments do not exacerbate the underlying disease. Once safety is confirmed, we will test the efficacy of our therapeutic tools against pathogens in the context of the pathology. This should provide sufficient data to establish a proof-of-concept in our mouse models and ex vivo human models for clinical development to improve respiratory function of severe asthmatics with little or no control on current therapies, and COPD patients.
Task 5.1- The IL-20 subfamily cytokines signal through different heterodimeric receptors. Team members have developed a unique tool targeting the IL-20Rb subunit – a neutralizing monoclonal human anti-IL-20Rb antibody that blocks signalling from both IL-20 receptors. We have previously shown this therapeutic tool to be effective against Streptococcus pneumoniae and PVM infections (data from TIILD members). We need to further investigate the impact of this unique translational tool in the context of pulmonary disorders.
Task 5.2- We recently identified the role of metabolites in modulating the mucosal immune response. Our goal is to manipulate the levels of these metabolites through modulating the microbiota using specific antibiotics or fecal material transfer (FMT).
The completion of the TIILD research project should lead to the following results. The TIILD team should (1) identify of the specific immune changes associated with each chronic lung disease that contribute to susceptibility to respiratory infections, (2) better understand the underlying mechanisms of immune dysfunction in the lung, providing avenues for the development of targeted immunomodulatory therapies, (3) validate immunological biomarkers that predict susceptibility to infection in patients with chronic lung disease, facilitating the development of personalised screening strategies, and finally (4) identify new therapeutic targets for the treatment of respiratory infections in patients with chronic lung disease, with the potential to improve their prognosis and quality of life.
Ultimately, by focusing on the defects of the mucosal immune response that predispose individuals to severe or recurrent pulmonary infections in asthma and COPD, the TIILD scientific project will contribute to the development of possible translational applications that correct these defects and alleviate the burden of pulmonary infections.
Current Staff
Saliha Ait-Yahia
PhD IR Inserm
Saliha's ORCID
Saliha's contact
Camille Audousset
MD, PhD, PH, Pulmonology (adult)
Camille's ORCID
Camille's contact
Cécile Chenivesse
MD, PhD, PU-PH Pulmonology (adult)
Cécile's ORCID
Cécile's conbtact
Silvia Demoulin
MD,PhD PU-PH Pulmonology (adult)
Silvia's ORCID
Silvia's contact
Rodrigue Dessein
MD, PhD, PU-PH Bacteriology
Rodrigue's ORCID
Rodrigue's contact
Emeline Driencourt
PhD
Emeline's contact
Charlotte Dufaye
IE
Emmanuel Faure
MD, PhD, PU-PH Infectious diseases
Emmanuel's ORCID
Emmanuel's contact
Karine Faure
MD, PhD, PU-PH Infectious diseases
Karine's ORCID
Karine's contact
Alexandre Gaudet
MD, PhD, MCU-PH Intensive care
Alexandre's ORCID
Alexandre's contact
Silvia Gaggero
CNRS researcher (CRCN)
Silvia's ORCID
Teddy Grandjean
IE ULille
Teddy's ORCID
Teddy's contact
Yasemine Karaca
MD,PhD
Angélina Kasprowicz
Post-doc
Angélina's ORCID
Gwenola Kervoaze
TE Inserm
Gwenola's contact
Eric Kipnis
MD, PhD, PU-PH Intensive care
Eric's ORCID
Eric's contact
Philippe Lassalle
INSERM researcher (CRCN), MD, PhD
Philippe's ORCID
Philippe's contact
Stéphanie Lejeune
MD, PhD, PU-PH Pulmonology (children)
Stéphanie's ORCID
Stéphanie's contact
Rémi Le Guern
MD, PhD, MCU-PH Bacteriology
Rémi's ORCID
Elise Leprêtre
AI
Elise's contact
Olivier Le Rouzic
MD, PU-PH Pulmonology (adult)
Olivier's ORCID
Olivier's contact
Layal Massara
Post-doc
Layal's ORCID
Marine Nouvel
PhD
Anais Ollivier
IE
Anais's contact
Erika Parmentier
MD PH Intensive care
Erika's ORCID
Erika's contact
Gaëtan Piga
MD
Gaëtan's ORCID
Gaëtan's contact
Odile Poulain
IPL researcher (CR)
Odile's ORCID
Odile's contact
Muriel Pichavant
INSERM researcher (CRCN)
Muriel's ORCID
Muriel's contact
Kevin Sermet
MD
Kevin's ORCID
Sarah Stabler
MD
The squalene route to C30 carotenoid biosynthesis and the origins of carotenoid biosynthetic pathways.
Santana-Molina C, Henriques V, Hornero-Méndez D, Devos DP, Rivas-Marin E. Proc Natl Acad Sci U S A. 2022 Dec 27;119(52):e2210081119. doi: 10.1073/pnas.2210081119. Epub 2022 Dec 19. PMID: 36534808
Reconciling Asgardarchaeota Phylogenetic Proximity to Eukaryotes and Planctomycetes Cellular Features in the Evolution of Life.
Devos DP. Mol Biol Evol. 2021 Aug 23;38(9):3531-3542. doi: 10.1093/molbev/msab186. PMID: 34229349
Origin and Evolution of Polycyclic Triterpene Synthesis.
Santana-Molina C, Rivas-Marin E, Rojas AM, Devos DP. Mol Biol Evol. 2020 Jul 1;37(7):1925-1941. doi: 10.1093/molbev/msaa054. PMID: 32125435
Non-essentiality of canonical cell division genes in the planctomycete Planctopirus limnophila.
Rivas-Marin E, Peeters SH, Claret Fernández L, Jogler C, van Niftrik L, Wiegand S, Devos DP. Sci Rep. 2020 Jan 9;10(1):66. doi: 10.1038/s41598-019-56978-8. PMID: 31919386
Essentiality of sterol synthesis genes in the planctomycete bacterium Gemmata obscuriglobus.
Rivas-Marin E, Stettner S, Gottshall EY, Santana-Molina C, Helling M, Basile F, Ward NL, Devos DP. Nat Commun. 2019 Jul 2;10(1):2916. doi: 10.1038/s41467-019-10983-7. PMID: 31266954